Using an insulin pump system made my life with diabetes so much easier
Heather
Medtronic Employee & Insulin Pump User
Why use Insulin Pump Therapy?
90%
Fewer injections
Lower
HbA1c5,6

What is Insulin Pump Therapy?
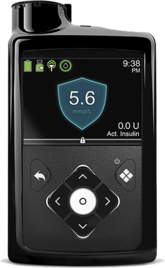
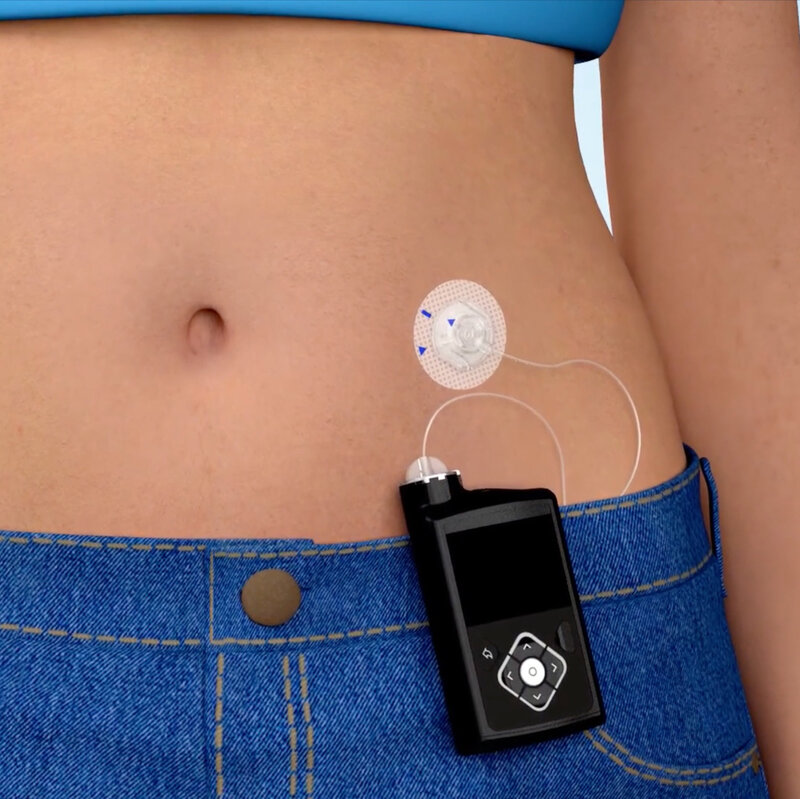
An insulin pump is a small device (about size of a smartphone) that mimics some of the ways a healthy pancreas works. It is safe, discreet and reliable. It can be attached to the same sites you currently inject in, and insulin is simply delivered to your body through a thin tube. An insulin pump replaces the need for frequent injections by delivering rapid acting insulin continuously 24 hours a day.

Who can use an Insulin Pump?
Talk to your healthcare professional to see if you’re eligible for insulin pump therapy:
Have type 1 diabetes and;
- Take three or more insulin injections per day
- Have received carbohydrate counting education
- Would like to better manage your diabetes
An insulin pump helps you to manage your diabetes. You will need to calculate your carbohydrate at mealtimes and the pump delivers tiny amounts of insulin in the background throughout the day, to replace your basal insulin.
Insulin Pump Therapy Benefits
Insulin pump therapy offers multiple clinical benefits over multiple daily injection therapy. With our pump and sensor system, you’re more likely to:
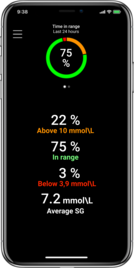
Keep your glucose levels in range1,2,3,4
Lower
HbA1c5,6
Higher time in
range1,2,5,8
Fewer Hypoglicaemic
events7
Live more,worry less
Less
injections
Worry less
about highs9
Worry less about low
during sleep9
Focus on your life,
not your glucose levels

The insulin pump makes decisions for me, and I now have more time to focus on what matter
Frequently asked questions
The device is safe, and can be used in all types of situations (sport, beach, going out) and insulin is simply delivered to your body through a thin tube. Thanks to smart accessories available today, it can be comfortably worn during school, exercise, formal occasions and everyday life.
Insulin pumps can be easily worn on or under your clothes very securely. The pump can also be detached for activities like swimming, showering and exercise so you can continue to live your life.
You can have your insulin pump with you whilst exercising. Temporary targets/basal rates are available (depending on pump model), plus MiniMed™ insulin pump systems are
It is small enough so it can be easily carried on a belt, in a pocket or even attached to a bra. The pump is about the same size as a pack of playing cards and similar in weight to an average lemon.
There are many ways your insulin pump can be worn. Whether at school, at work,at a formal occasions or just in everyday life, your insulin pumps can be easily worn on or under your clothes very securely. The pump can also be detached for activities like contact sport, showering and exercise.
MiniMed™ insulin pumps are
- Carlson, A.L. et al. 97-P- Safety and glycaemic outcomes of the MiniMed™ AHCL System in subjects with T1D. 80th ADA International Conference, June 2020, Chicago
- Collyns .O. et al. 199-OR- Improved glycaemic Outcomes with MiniMed™ AHCL Delivery. 80th ADA International Conference, June 2020, Chicago
- Agiostratidou, G. et al. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017; 40(12):1622–1630.
- Danne T. et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017; 40(12):1631-1640.
- Bergenstal, R. M. et al.Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes Jama. 2016; 316 (13): 1407 – 1408
- J. C. Pickup and A. J. Sutton Severe hypoglycaemia and glycemic control in Insulin Dependant Diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion Diabetic Medicine 2008 :25, 765–774.
- Bergenstal RM1, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Perkins BA, Welsh JB, Willi SM, Wood MA; STAR 3 Study Group. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011 Nov;34(11):2403-5. doi: 10.2337/dc11-1248. Epub 2011 Sep 20.
- Assumes four injections per day for 30 days and one infusion set change every three days.
- Medtronic data on file. Pivotal Trial (Age 14-75). N=157. 2020; 16 US sites.
- 1.Carlson, A.L. et al. 97-P- Safety and glycaemic outcomes of the MiniMed™ AHCL System in subjects with T1D. 80th ADA International Conference, June 2020, Chicago
- 2.Collyns .O. et al. 199-OR- Improved glycaemic Outcomes with MiniMed™ AHCL Delivery. 80th ADA International Conference, June 2020, Chicago
- 3.Agiostratidou, G. et al. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017; 40(12):1622–1630.
- 4.Danne T. et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017; 40(12):1631-1640.
- 1.Carlson, A.L. et al. 97-P- Safety and glycaemic outcomes of the MiniMed™ AHCL System in subjects with T1D. 80th ADA International Conference, June 2020, Chicago
- 2.Collyns .O. et al. 199-OR- Improved glycaemic Outcomes with MiniMed™ AHCL Delivery. 80th ADA International Conference, June 2020, Chicago
- 5.Bergenstal, R. M. et al.Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes Jama. 2016; 316 (13): 1407 – 1408
- 1.Carlson, A.L. et al. 97-P- Safety and glycaemic outcomes of the MiniMed™ AHCL System in subjects with T1D. 80th ADA International Conference, June 2020, Chicago
- 2.Collyns .O. et al. 199-OR- Improved glycaemic Outcomes with MiniMed™ AHCL Delivery. 80th ADA International Conference, June 2020, Chicago
- 5.Bergenstal, R. M. et al.Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes Jama. 2016; 316 (13): 1407 – 1408
- 8.Assumes four injections per day for 30 days and one infusion set change every three days.
- 5.Bergenstal, R. M. et al.Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes Jama. 2016; 316 (13): 1407 – 1408
- 6.Assumes four injections per day for 30 days and one infusion set change every three days.
- 7.Bergenstal RM1, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Perkins BA, Welsh JB, Willi SM, Wood MA; STAR 3 Study Group. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011 Nov;34(11):2403-5. doi: 10.2337/dc11-1248. Epub 2011 Sep 20.
- 9.Medtronic data on file. Pivotal Trial (Age 14-75). N=157. 2020; 16 US sites.
- † Assumes four injections per day for 30 days and one infusion set change every three days
- * Compared to MiniMed™ 670G system
- ** For MiniMed™ 700 series devices only: At time of manufacture and when the reservoir and tubing are properly inserted, your pump is waterproof. It is protected against the effects of being underwater to a depth of up to 2.4 Meters (8 feet) for up to 30 minutes. This is classified as IPX8 rating. See user guide for details.