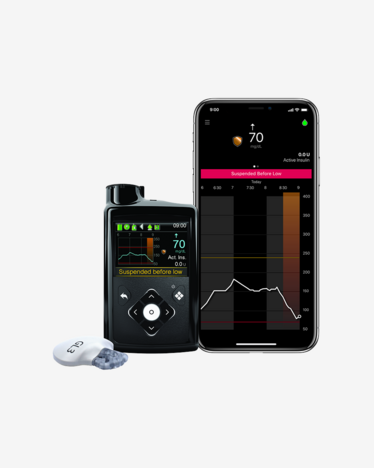
Do you know the real benefit of using a CGM integrated with your insulin pump?

Smart Guard Technology
Measures your glucose levels every 5 minutes 24/7 with a different insulin delivery automation setting to help preventing your blood glucose from going too low and too
- Collyns OJ, et al. Poster # 979 at the 80th International Conference of the American Diabetes Association, June 2020, Chicago/Virtual
- Bosi E, et al. SMILE Study Group. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7(6):462-472
- Da Silva J, et al. Realworld performance of the MiniMed™ 670G system in Europe. Diabetes Obes Metab. 2021;23(8):1942-1949.
- Da Silva J, et al. Real-world Performance of the MiniMed™ 780G System: First Report of Outcomes from 4'120 Users. Diabetes Technol Ther. 2021 Sep 15. doi: 10.1089/dia.2021.0203.
- Bergenstal, R. M. et al. Jama. 2016; 316 (13): 1407 – 1408.
- Garg SK et al. Diabetes Technol Ther. 2017 Mar;19(3):155-163.
- * Compared vs the MiniMed™ 670G system. Refer to System User Guide - SmartGuard ™ feature. Some user interaction required.
- ** A BG needed when you first enter SmartGuard ™ feature. If glucose alerts and CGM readings do not match your symptoms, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide
- *** Compared to CSII
- # Glucose Management Indicator (GMI)
- 1.Collyns OJ, et al. Poster # 979 at the 80th International Conference of the American Diabetes Association, June 2020, Chicago/Virtual
- 2.Bosi E, et al. SMILE Study Group. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7(6):462-472
- 3.Da Silva J, et al. Realworld performance of the MiniMed™ 670G system in Europe. Diabetes Obes Metab. 2021;23(8):1942-1949.
- 4.Da Silva J, et al. Real-world Performance of the MiniMed™ 780G System: First Report of Outcomes from 4'120 Users. Diabetes Technol Ther. 2021 Sep 15. doi: 10.1089/dia.2021.0203.
- 2.Bosi E, et al. SMILE Study Group. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7(6):462-472
- 3.Da Silva J, et al. Realworld performance of the MiniMed™ 670G system in Europe. Diabetes Obes Metab. 2021;23(8):1942-1949.
- 4.Da Silva J, et al. Real-world Performance of the MiniMed™ 780G System: First Report of Outcomes from 4'120 Users. Diabetes Technol Ther. 2021 Sep 15. doi: 10.1089/dia.2021.0203.
- 5.Bergenstal, R. M. et al. Jama. 2016; 316 (13): 1407 – 1408.
- 6.Garg SK et al. Diabetes Technol Ther. 2017 Mar;19(3):155-163.
This material does not replace or supersede the instructions for use. It should not be considered the exclusive source of information, and should be used in conjunction with the User Guide. See the User Guide for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events. For further information, contact your local Medtronic representative and/or consult the Medtronic website at medtronic.eu.
Information contained herein is not medical advice and should not be used as an alternative to speaking with your doctor. Discuss indications, contraindications, warnings, precautions, potential adverse events and any further information with your health care professional.