
Better diabetes control without the worry of going low1,2
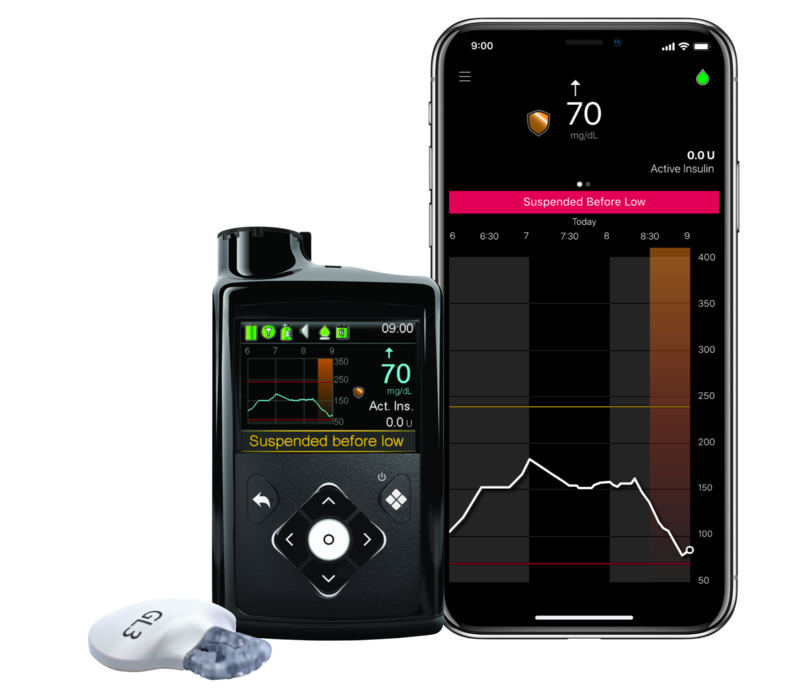
MiniMed™ 740G system
The smart device compatible insulin pump that predicts and prevents lows before you feel
MiniMed™ 740G system can help prevent 80% of lows1
Takes actions to prevent lows.
Achieving glucose control often means worrying about finding ways to bring high glucose levels back in range – without increasing your chances of going low. SmartGuard™ feature helps take those worries away by preventing up to 80% of lows without increasing the risk of
Less time worrying about nighttime lows.
With SmartGuard™ tech, you can set multiple low limits throughout the day or night. Insulin is suspended before the preset low level to help prevent lows, so you have more time in control than ever
Sensor augmented pump can help to lower your HBA1C3 :
Up to 1.0% HbA1c
MiniMed™ 740G with SmartGuard™ tech is designed to take action to prevent you from going low.
System continuously checks your sensor glucose levels every 5 minutes, day and night, to predict and prevent lows 30 minutes ahead of time. SmartGuard™ tech will automatically stop insulin delivery before you go low and will resume on its own when your levels recover to give you more control over your diabetes
SmartGuard™ technology
SmartGuard™ feature helps prevent lows to give you more control1
1 SmartGuard™ Feature suspends insulin when sensor glucose (SG) is predicted to go low
2 SmartGuard™ Feature resumes basal insulin when (SG) recovers
Insulin suspended window (suspended up to 2 hours)
SG Reading
- Choudhary P, et al. Hypoglycemia Prevention and User Acceptance of an Insulin Pump System with Predictive Low Glucose Management Diabetes Technol Ther. 2016; 18(5):288-291
- Bosi E, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019;7: 462–72
- Bergenstal RM, Effectiveness of Sensor-Augmented Insulin-Pump Therapy in Type 1 Diabetes. New England Journal of Medicine. 2010;363(11):1092-1092
- Biester T. et al. ‘‘Let the Algorithm Do the Work’’: Reduction of Hypoglycemia Using Sensor- Augmented Pump Therapy with Predictive Insulin Suspension (SmartGuard) in Pediatric Type 1 Diabetes Patients. Diabetes Technol Ther. 2017; 19(3):173-182
- 1.Choudhary P, et al. Hypoglycemia Prevention and User Acceptance of an Insulin Pump System with Predictive Low Glucose Management Diabetes Technol Ther. 2016; 18(5):288-291
- 2.Bosi E, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019;7: 462–72
- 1.Choudhary P, et al. Hypoglycemia Prevention and User Acceptance of an Insulin Pump System with Predictive Low Glucose Management Diabetes Technol Ther. 2016; 18(5):288-291
- 2.Bosi E, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019;7: 462–72
- 1.Choudhary P, et al. Hypoglycemia Prevention and User Acceptance of an Insulin Pump System with Predictive Low Glucose Management Diabetes Technol Ther. 2016; 18(5):288-291
- 2.Bosi E, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019;7: 462–72
- 3.Bergenstal RM, Effectiveness of Sensor-Augmented Insulin-Pump Therapy in Type 1 Diabetes. New England Journal of Medicine. 2010;363(11):1092-1092
- 3.Bergenstal RM, Effectiveness of Sensor-Augmented Insulin-Pump Therapy in Type 1 Diabetes. New England Journal of Medicine. 2010;363(11):1092-1092
- 4.Biester T. et al. ‘‘Let the Algorithm Do the Work’’: Reduction of Hypoglycemia Using Sensor- Augmented Pump Therapy with Predictive Insulin Suspension (SmartGuard) in Pediatric Type 1 Diabetes Patients. Diabetes Technol Ther. 2017; 19(3):173-182
- 5.Medtronic data on file: MiniMed™ 780G users survey conducted in April-May 2021 in UK, Sweden, Italy, Netherlands and Belgium. Sample size = 789.
- 9.Zhang G, et al. 986-P at the 80th ADA International Conference, June 2020.
- 10.Ilany J, et al. Abstract 416 at the 13th ATTD International Conference. February 2020.
- * MiniMed™ 740G and the MiniMed™ 640G system share the same therapy algorithm.
- ** Glucose Management Indicator (GMI) data from CGM values were used to estimate HbA1c. Calculated using JAEB https://www.jaeb.org/gmi/.
- *** Compared with the MiniMed™ 670G system.
- † A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions.
- †† Refer to MiniMed™ Mobile app. User Guide
- ††† At the time of manufacture and when the reservoir and tubing are properly inserted, your pump is waterproof.
- # Multiple Daily Injections (3 bolus and 1 basal insulin per day) requires 28 injections per week vs. 1 with Medtronic Extended Infusion Set.
- ## Versus SAP+PLGM or pre-AHCL initiation.
- ### vs the 3-day infusion set MiniMed™ Quick-set™
- ~ When used with Medtronic Extended infusion set. Refer to User Guide.
- * Refer to System User Guide - SmartGuard™ feature. Some user interaction required.
- † A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions.
- 10.Ilany J, et al. Abstract 416 at the 13th ATTD International Conference. February 2020.
- ~ When used with Medtronic Extended infusion set. Refer to User Guide.
- For illustrative purposes only.
UC202200109 EE ©2021 Medtronic. All rights reserved. Medtronic and the Medtronic logo are trademarks of Medtronic. TM* Third party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. ACCU-CHECK AND ACCU-CHEK GUIDE LINK are trademarks of Roche Diabetes Care. The legal manufacturer of MiniMed™ infusion sets is Unomedical a/s. Aaholmvej 1-3, Osted. 4320 Lejre, Denmark.